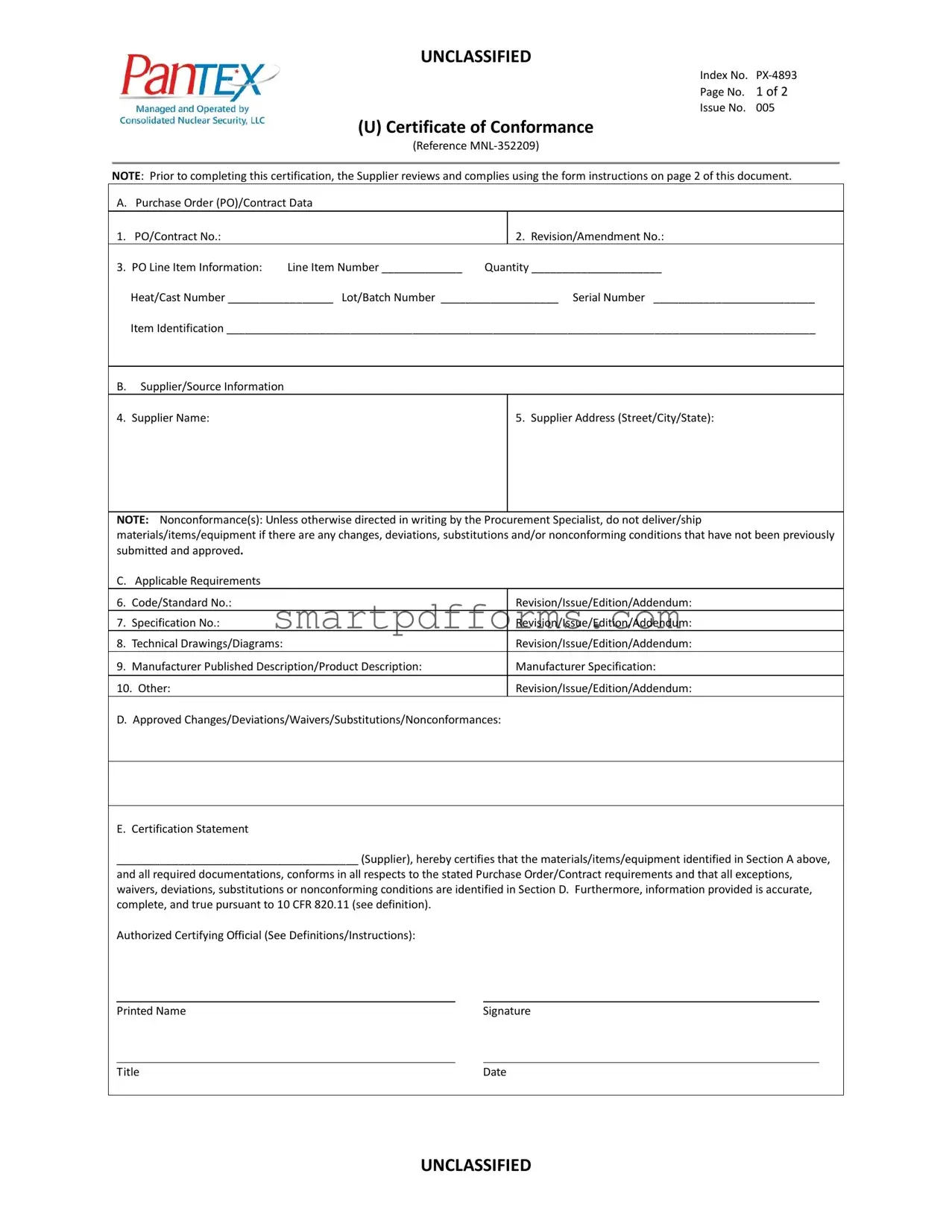
Blank Certificate Of Conformance PDF Template
In the realm of supplier and procurement relationships, maintaining assurance in the quality and compliance of products and materials is essential. The Certificate of Conformance, as detailed in the referenced document MNL-352209, serves as a critical tool in this assurance process. This document, primarily issued by suppliers to assert that their delivered goods or services meet the required specifications laid out in a purchase order or contract, covers several key areas to ensure transparency and compliance. It includes detailed information on the purchase order or contract, such as the order number, revision or amendment numbers, and specific item identification details like quantity, heat/cast number, lot/batch number, and serial number. Additionally, it encompasses supplier/source information, highlighting the supplier's legal name and addressing details. It mandates the inclusion of applicable requirements regarding design codes, standards, technical drawings, product descriptions, and other relevant documents. Furthermore, it addresses the protocol for deviations, waivers, substitutions, or any nonconformance issues, requiring these to be approved and documented. The certification statement at the end of the document is a declaration by the supplier, asserting the full compliance of the goods or services with the order requirements and the accuracy of the information provided. This certification not only facilitates trust between suppliers and purchasers but also adheres to regulatory compliance, specifically referencing procedural rules for DOE nuclear activities (10 CFR 820.11). This form, integral to the quality assurance process, ensures that products or services provided adhere strictly to the agreed-upon specifications and standards.
Preview - Certificate Of Conformance Form
UNCLASSIFIED
Index No.
Page No. 1 of 2
Issue No. 005
(U) Certificate of Conformance
(Reference
NOTE: Prior to completing this certification, the Supplier reviews and complies using the form instructions on page 2 of this document.
A. Purchase Order (PO)/Contract Data
1. |
PO/Contract No.: |
|
|
2. Revision/Amendment No.: |
3. |
PO Line Item Information: |
Line Item Number _____________ |
Quantity _____________________ |
|
|
Heat/Cast Number _________________ Lot/Batch Number ___________________ Serial Number __________________________ |
|||
|
Item Identification _______________________________________________________________________________________________ |
|||
|
|
|
|
|
B. |
Supplier/Source Information |
|
|
|
4. |
Supplier Name: |
|
|
5. Supplier Address (Street/City/State): |
NOTE: Nonconformance(s): Unless otherwise directed in writing by the Procurement Specialist, do not deliver/ship materials/items/equipment if there are any changes, deviations, substitutions and/or nonconforming conditions that have not been previously submitted and approved.
C. |
Applicable Requirements |
|
6. |
Code/Standard No.: |
Revision/Issue/Edition/Addendum: |
7. |
Specification No.: |
Revision/Issue/Edition/Addendum: |
8. |
Technical Drawings/Diagrams: |
Revision/Issue/Edition/Addendum: |
9. |
Manufacturer Published Description/Product Description: |
Manufacturer Specification: |
10. Other: |
Revision/Issue/Edition/Addendum: |
|
D. Approved Changes/Deviations/Waivers/Substitutions/Nonconformances:
E. Certification Statement
_______________________________________ (Supplier), hereby certifies that the materials/items/equipment identified in Section A above,
and all required documentations, conforms in all respects to the stated Purchase Order/Contract requirements and that all exceptions, waivers, deviations, substitutions or nonconforming conditions are identified in Section D. Furthermore, information provided is accurate, complete, and true pursuant to 10 CFR 820.11 (see definition).
Authorized Certifying Official (See Definitions/Instructions):
Printed Name |
|
Signature |
|
|
|
Title |
|
Date |
UNCLASSIFIED

UNCLASSIFIED
Index No.
Page No. 2 of 2
Issue No. 005
(U) Certificate of Conformance
(Reference
Instructions
Prepare a Certificate of Conformance (C of C) addressing each PO line item, Contract Deliverable, or each partial shipment. Unless otherwise specified, the C of C accompanies each shipment. All applicable form entries are completed.
A Supplier
Definitions
Authorized Certifying Official - The certification is attested to by an authorized representative of the supplier; and the certification system, including the procedures for completing, reviewing, and approving the certificate are described in the Company’s administrative control system or Quality Assurance program.
Certification - The act of determining, verifying, and attesting in writing to the qualifications of personnel, processed, procedures, or items in accordance with specified requirements.
Certificate of Conformance - A document signed or otherwise authenticated by an authorized individual certifying the degree to which items or services meet specified requirements.
10 CFR 820.11 - Procedural Rules for DOE Nuclear Activities, Subpart “A”, Information requirements. The regulation states: Any information pertaining to a nuclear activity provided to DOE by any person or maintained by any person for inspection by DOE are complete and accurate in all material respects.
No person involved in a DOE nuclear activity conceals or destroys any information concerning a violation of a DOE Nuclear Safety Requirement, a Nuclear Statute, or the Act.
Section A, Purchase Order (PO)/Contract Data
Entry 1 Enter the complete CNS Pantex PO or Contract Number.
Entry 2 Enter PO/Contract Revision or Amendment Number, (if applicable).
Entry 3 Enter as applicable, the PO Line Item Number (i.e., 1, 2, 3), quantity, heat/cast number, lot/batch number, serial number, and item identification.
Section B, Supplier/Source Information
Entry 4 Enter the legal Supplier company name, as stated on the PO or Contract.
Entry 5 Enter the Supplier business address, as stated on the PO or Contract.
Section C, Applicable Requirements
Entry 6 Enter the applicable design code/standard number and applicable revision, issue, edition, or addendum.
Entry 7 Enter the applicable specification number and applicable revision, issue, edition, or addendum.
Entry 8 Enter the applicable technical drawing/diagram and applicable revision, issue, edition, or addendum.
Entry 9 Mark the applicable box manufacturer published description/product description or manufacturers specification.
Entry 10 Enter other applicable requirements documents and applicable revision, issue, edition, or addendum.
Section D, Approved Changes/Deviations/Waivers/Substitutions/Nonconformances
Enter any approved changes. Reference change documentation control numbers as applicable. (Attach additional pages if necessary).
Section E, Certification Statement (see definitions)
Enter the Company name (or commonly used acronym).
Print or type the authorized company certifying officials name, title, and date.
Sign or otherwise authenticate by company certifying official.
Transmittal:
CNS Pantex, LLC
Fax:
Attn: SUPPLIER QUALITY
Or
Place a copy with the shipment.
UNCLASSIFIED
Form Data
| Fact Name | Description |
|---|---|
| Purpose | The Certificate of Conformance ensures that materials, items, or equipment meet the specified requirements detailed in the Purchase Order or Contract. |
| Content Requirement | Includes PO/Contract data, supplier/source information, applicable requirements, approved changes/deviations, and a certification statement. |
| Governing Law | The form is governed by 10 CFR 820.11, which mandates that information related to DOE nuclear activities be complete and accurate. |
| Submission Guidelines | The completed Certificate of Conformance must accompany each shipment or partial shipment and can be submitted via fax or email as detailed in the form instructions. |
Instructions on Utilizing Certificate Of Conformance
Filling out a Certificate of Conformance form might initially seem daunting, but it's actually a straightforward process if you break it down step by step. This document is vital for ensuring that materials, items, or equipment meet all the necessary requirements and standards outlined in a purchase order or contract. It's a guarantee to your clients that you're delivering what you've promised, in compliance with all relevant specifications. Here's how to navigate the completion of this form, ensuring every detail is accurately captured to avoid any potential hiccups in your delivery process.
- Section A, Purchase Order (PO)/Contract Data:
- Enter the complete CNS Pantex PO or Contract Number in the provided space.
- Include the PO/Contract Revision or Amendment Number, if applicable.
- For each PO Line Item, enter the Line Item Number, quantity, heat/cast number, lot/batch number, serial number, and item identification as applicable.
- Section B, Supplier/Source Information:
- Enter the legal name of your company, as stated on the PO or Contract.
- Provide the business address of your company, exactly as stated on the PO or Contract.
- Section C, Applicable Requirements:
- Enter the appropriate design code/standard number along with its revision, issue, edition, or addendum.
- Provide the applicable specification number and its revision, issue, edition, or addendum.
- Specify the relevant technical drawing/diagram and its revision, issue, edition, or addendum.
- Mark the box that pertains to you - either manufacturer published description/product description or manufacturer's specification.
- For "Other" requirements, enter any additional documents along with their applicable revision, issue, edition, or addendum.
- Section D, Approved Changes/Deviations/Waivers/Substitutions/Nonconformances: Enter any approved changes and reference their documentation control numbers, attaching additional pages if necessary.
- Section E, Certification Statement: Fill in your company's name (or a commonly used acronym). Print or type the authorized company certifying official's name, title, and date. Then, have the company certifying official sign or otherwise authenticate the form.
After you've completed all the sections of the Certificate of Conformance form, remember to either fax it to CNS Pantex, LLC at 806-477-3891 or email a PDF file to supplierquality@pantex.com. It's also crucial to include a copy of the certificate with your shipment. By meticulously following these steps, you'll help ensure that your materials, items, or equipment clear the necessary compliance hurdles, paving the way for a successful delivery.
Obtain Answers on Certificate Of Conformance
What is a Certificate of Conformance?
A Certificate of Conformance is a document that an authorized individual signs or authenticates, demonstrating that items or services meet specified requirements. It serves as a declaration that the products or services provided adhere to the agreed specifications, codes, standards, and other necessary criteria outlined in a Purchase Order or Contract.
Who can issue a Certificate of Conformance?
The document must be issued by an authorized certifying official. This official is a representative of the supplier, designated as responsible for determining, verifying, and attesting the conformance of materials or services. The certifying system, including procedures for completing, reviewing, and approving the certificate, should be part of the company's administrative control system or Quality Assurance program.
When should a Certificate of Conformance be prepared?
A Certificate of Conformance must be prepared for each Purchase Order line item, Contract Deliverable, or each partial shipment. Unless specified otherwise, the certificate should accompany each shipment. It's essential to ensure that all applicable form entries are completed and that a system-generated Certificate of Conformance from the supplier, which contains all required information, can be attached and referenced.
What information is required on a Certificate of Conformance?
The certificate must include:
- Purchase Order or Contract data including the number, any revision or amendment number, line item information such as quantity, heat/cast number, lot/batch number, serial number, and item identification.
- Supplier or source information like the legal company name and the business address.
- Applicable requirements including the design code/standard number, specification number, technical drawings/diagrams, and any other necessary documents along with their revisions or editions.
- Any approved changes, deviations, waivers, substitutions, or nonconformances.
- A certification statement from the supplier, certifying that the products or services conform to the stated requirements and noting any exceptions.
How should a Certificate of Conformance be submitted?
The completed certificate can be transmitted via fax to CNS Pantex, LLC at the specified number or emailed as a PDF file to the provided supplier quality email address. A copy of the certificate should also be included with the shipment to ensure that all relevant parties can verify the conformance of the delivered items or services.
What regulations govern the accuracy of a Certificate of Conformance?
Regulation 10 CFR 820.11 outlines procedural rules for DOE (Department of Energy) nuclear activities. It mandates that any information provided to the DOE concerning nuclear activities must be complete and accurate in all material respects. This regulation ensures that the certificate of conformance upholds a high standard of integrity, as inaccuracies or the concealment of violations could have significant safety implications.
Common mistakes
When filling out the Certificate of Conformance form, careful attention to detail is crucial. Here are four common mistakes that people often make:
- Not reviewing the form instructions thoroughly. The second page of the document provides essential guidelines on how to accurately complete the form. Skipping this step often leads to errors or omissions in the form entries.
- Failing to provide complete Purchase Order (PO)/Contract Data. Section A requires detailed information about the PO/Contract, including the PO/Contract No., Revision/Amendment No., and specific line item details. Missing or inaccurate information in this section can result in non-conformance of the documentation.
- Omitting approved changes, deviations, waivers, substitutions, or nonconformances. Section D is crucial for identifying any variations from the original PO/Contract requirements. Neglecting to detail these items can invalidate the certificate, as it assures the buyer that all specifications have been met, including any agreed-upon changes.
- Inaccurate or incomplete certification statement. The certification statement in Section E is a legal attestation of conformity. Incorrectly filling out the supplier name, or failing to include the printed name, signature, title, and date by an authorized certifying official, compromises the document's validity.
Addressing these common mistakes involves:
- Reading all instructions carefully before proceeding.
- Double-checking all entries against the PO/Contract documentation to ensure information is complete and accurate.
- Detailing any exceptions or modifications explicitly in the form and attaching additional documentation if necessary.
- Ensuring that the certification statement is accurately filled out and duly signed by an authorized individual.
This cautious approach ensures that the Certificate of Conformance accurately reflects compliance with all contractual requirements, thereby minimizing potential disputes or rejections based on documentation errors.
Documents used along the form
Acquiring a Certificate of Conformance is a critical step in ensuring the quality and compliance of products and services within various industries. However, this document does not stand alone in the certification and procurement process. Several other forms and documents often accompany the Certificate of Conformance to provide a comprehensive overview of a product's compliance and specifications. Understanding each of these documents helps in navigating the legal and regulatory landscape efficiently.
- Purchase Order (PO): Specifies the details of the products or services ordered by the buyer, including quantity, price, and delivery schedule.
- Material Safety Data Sheet (MSDS): Provides detailed information about the handling, storage, and safety measures for chemicals or hazardous materials.
- Inspection Report: Documents the findings of a detailed examination of the product or service to ensure it meets specified standards and requirements.
- Quality Assurance Plan: Outlines the processes and procedures a supplier uses to maintain quality standards throughout production or service delivery.
- Test Report: Contains results from tests conducted to ensure the product meets the required specifications and standards.
- Shipping and Packing List: Details the contents of a shipment, including types and quantities of products, and provides instructions for handling and packing.
- Warranty Document: Guarantees the condition of the product and outlines the terms for repair or replacement if it fails to meet stated requirements.
- Technical Drawings: Provides detailed graphical and textual representations of a product, highlighting its dimensions, materials, and functional specifications.
Including these forms and documents with a Certificate of Conformance ensures a seamless and transparent procurement process. It provides all parties involved with a clear understanding of product specifications, safety measures, and quality assurance practices, facilitating trust and compliance with industry regulations. Thus, it's essential for suppliers and buyers alike to familiarize themselves with these documents to ensure a comprehensively documented transaction that meets all applicable standards and requirements.
Similar forms
Certificate of Analysis (CoA): Like the Certificate of Conformance, a Certificate of Analysis provides validation for products, specifically focusing on their chemical and physical properties. It verifies that product batches meet the specified criteria, often including detailed test results for additional assurance to purchasers about the quality and compliance of the products.
Inspection Certificate: This document is similar because it also serves as proof that goods have been inspected and meet the required quality standards or specifications set by the buyer or regulatory bodies. While a Certificate of Conformance generally declares conformity with specifications, an Inspection Certificate often contains specific findings and measurements taken from the inspected goods.
Manufacturer's Certificate of Compliance (MCC): The MCC is another document that parallels the Certificate of Conformance in purpose. It certifies that a product meets all regulatory, market, or consumer expectations. It typically includes manufacturer details, information on the standards met, and possibly the results from tests conducted to verify compliance.
Quality Assurance Certificate: This certification reassures the receiver of the goods that all quality assurance measures have been taken to ensure the product meets the outlined specifications and standards. Similar to the Certificate of Conformance, it is a statement of quality, but with more focus on the processes and systems in place to maintain quality standards.
Material Test Report (MTR): Whereas the Certificate of Conformance provides a general declaration that products conform to specified standards and requirements, a Material Test Report offers in-depth details of the chemical and mechanical properties of materials used in the production of these goods. It is often used in industries where material composition is critical, making it a more detailed counterpart to the broader conformance certification.
Dos and Don'ts
When you're tasked with filling out a Certificate of Conformance form, it's crucial to handle the process meticulously to guarantee that the final document accurately reflects compliance with all specified requirements. To ensure that you navigate this process smoothly, here are several dos and don'ts:
- Do review and understand the instructions on page 2 of the Certificate of Conformance form before beginning to fill it out. This initial step is critical for ensuring that all subsequent actions align with the specified guidelines.
- Do verify that all information entered on the form is complete, accurate, and reflective of the current status of the materials, items, or equipment being certified. Inaccuracies can lead to noncompliance issues.
- Do ensure that you have legal authority or have been designated as the authorized certifying official before signing the certification statement. This responsibility carries legal obligations.
- Do include any approved changes, deviations, waivers, substitutions, or nonconformances in Section D as specified since transparency in these areas is crucial for the integrity of the certification.
- Don't skip any required fields. If a section does not apply, make sure to note that appropriately instead of leaving it blank. This approach avoids confusion and ensures that the review process can proceed smoothly.
- Don't proceed with delivery or shipment if there are any known nonconformances or deviations that haven't been officially submitted and approved. This can have significant legal and contractual repercussions.
- Don't hesitate to attach additional pages if necessary, especially in the case of approved changes or deviations that require comprehensive documentation. Make sure these attachments are clearly referenced within the form.
- Don't forget to include a copy of the Certificate of Conformance with the shipment and to also send it through the required channels, whether via fax or email, as outlined. Proper transmission is essential for the documentation to be recognized and accepted.
Navigating the nuances of a Certificate of Conformance requires attention to detail and an understanding of the form's requirements. By following these guidelines, you can ensure that your documentation accurately reflects compliance and supports the integrity of the delivery process.
Misconceptions
When discussing the Certificate of Conformance, several misconceptions frequently arise that can lead to misunderstandings about its purpose, requirements, and implications. Here's a closer look at some of these misconceptions:
Misconception 1: The Certificate of Conformance is merely a formality without any real importance.
Misconception 2: All sections of the Certificate of Conformance must be filled out by the supplier alone, without any input or assistance from other parties.
Misconception 3: The Certificate of Conformance serves the same purpose across all industries without adaptation to context-specific guidelines.
Misconception 4: Once issued, the Certificate of Conformance cannot be amended or updated to reflect changes or corrections.
Misconception 5: Compliance with the Certificate of Conformance guarantees that a product meets all possible quality and safety standards universally.
Misconception 6: There are no legal consequences for inaccuracies or fabrications made on a Certificate of Conformance.
Misconception 7: The Certificate of Conformance is an internal document only and is not meant for review by customers or regulatory authorities.
Misconception 8: Any employee of the supplier can sign the Certificate of Conformance, regardless of their position or level of authority.
Each of these misconceptions can lead to potential pitfalls for both suppliers and purchasers. For instance, underestimating the document's significance could result in non-compliance with contract specifications or legal regulations, leading to possible legal action or financial loss. Understanding that the Certificate of Conformance is a crucial document evidencing compliance with specified requirements and that it involves responsibilities and potential liabilities is essential for all parties involved in the supply chain. Information must be accurate and comprehensive, reflecting any approved deviations or nonconformances. Additionally, the signatory must be an authorized certifying official as per company policy and regulatory standards. This underscores the importance of ensuring that the process for issuing a Certificate of Conformance is taken with the seriousness and precision it warrants.
Key takeaways
When preparing a Certificate of Conformance (C of C), it is essential to meticulously review and adhere to the guidelines provided on page 2 of the document.
Each Purchase Order (PO) line item, Contract Deliverable, or partial shipment must have an accompanying Certificate of Conformance, unless otherwise specified. This ensures that the items shipped meet the agreed-upon requirements.
It is mandatory to complete all applicable sections of the form. Failure to include necessary information may result in the rejection of the shipment or delay in processing.
The Supplier's legal name and business address must be accurately recorded in the certification. This information should match what was stated on the purchase order or contract to avoid any discrepancies.
Specifications, design codes, and technical drawings relevant to the purchase order or contract must be detailed in the Certificate of Conformance, including the appropriate issue, revision, or edition numbers.
Any approved changes, deviations, waivers, substitutions, or nonconformances related to the shipped items must be clearly identified in the provided sections. This transparency is crucial for maintaining quality and compliance standards.
The certificate must be certified by an authorized official of the supplier company, verifying that the shipped items fully comply with the contract specifications. This includes signing or otherwise authenticating the document.
Suppliers can attach a system-generated Certificate of Conformance as long as it contains all the information required by the original form. This can streamline the process by using existing documents.
All information provided on the Certificate of Conformance must be complete and accurate, meeting the regulatory requirements outlined in 10 CFR 820.11. This ensures integrity and reliability in the supply chain for nuclear activities.
Popular PDF Forms
How Do I Know If My Medical Is Active - It ensures that every family member's health and dental care needs are considered under the Medi-Cal program.
Gcaar Residential Lease Washington Dc - Clarifies the non-refundable nature of application fees used for processing, including credit checks.
Notice of Appeal Illinois - Appellants are reminded of their responsibility to comply with IDES Administrative Rule 2720.315(b), focusing on the proper introduction and certification of new evidence.