Blank Cms 847 PDF Template
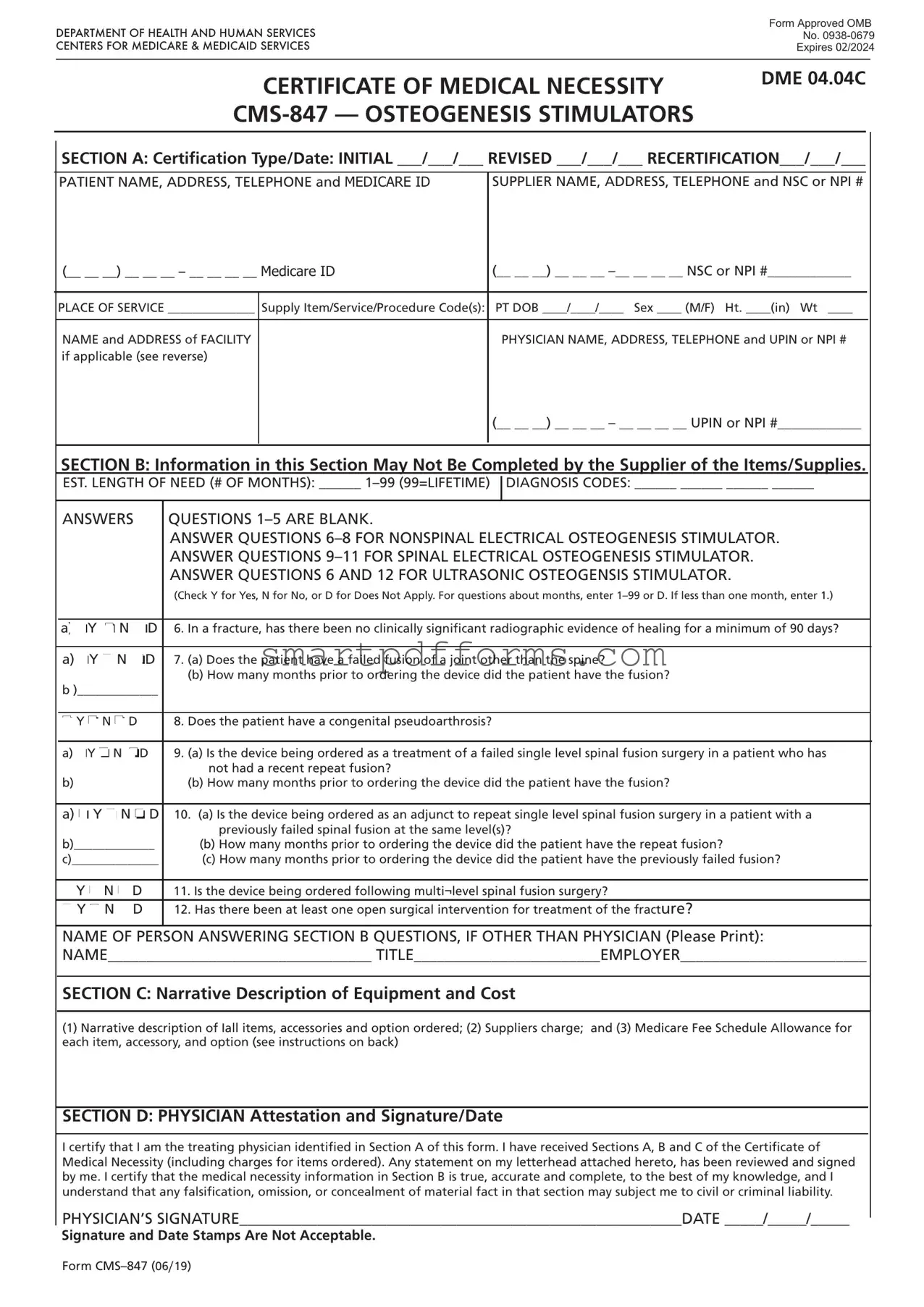
The CMS-847 form plays a critical role in the healthcare industry, serving as a Certificate of Medical Necessity for Osteogenesis Stimulators. Approved by the Office of Management and Budget and mandated by the Centers for Medicare & Medicaid Services, this document, with an expiration date in February 2024, ensures that patients receive medically necessary devices for bone growth stimulation. The form is structured into several sections, designed to capture comprehensive information necessary for the approval of these devices. Section A focuses on certification type and date, along with patient and supplier information, establishing the initial context and need. Details about the patient, including address and Medicare ID, alongside the supplier's information, underpin the form's relevance to specific individuals. Notably, Section B calls for detailed medical justifications for the osteogenesis stimulator, relying on clinical information about the patient's condition and the expected duration of the device's necessity. It emphasizes the requirement for a clinician's insight, barring suppliers from directly completing this section. The document further extends into Section C, which outlines the equipment and its costs, and Section D, which includes a declaration by the treating physician, affirming the authenticity and medical necessity of the information provided. This structured form reflects a bureaucratic yet essential process aimed at affirming and substantiating the medical necessity of osteogenesis stimulators, ensuring that patients have access to crucial supportive care under Medicare provisions.
Preview - Cms 847 Form
Form Approved OMB
DEPARTMENT OF HEALTH AND HUMAN SERVICES |
No. |
|
CENTERS FOR MEDICARE & MEDICAID SERVICES |
Expires 02/2024 |
|
|
|
|
CERTIFICATE OF MEDICAL NECESSITY
DME 04.04C
SECTION A: Certification Type/Date: INITIAL ___/___/___ REVISED ___/___/___ RECERTIFICATION___/___/___
PATIENT NAME, ADDRESS, TELEPHONE and MEDICARE ID |
SUPPLIER NAME, ADDRESS, TELEPHONE and NSC or NPI # |
(__ __ __) __ __ __ – __ __ __ __ Medicare ID |
(__ __ __) __ __ __ |
|
|
|
|
PLACE OF SERVICE ______________ |
Supply Item/Service/Procedure Code(s): |
PT DOB ____/____/____ Sex ____ (M/F) Ht. ____(in) Wt ____ |
|
|
|
NAME and ADDRESS of FACILITY |
|
PHYSICIAN NAME, ADDRESS, TELEPHONE and UPIN or NPI # |
if applicable (see reverse) |
|
|
|
|
(__ __ __) __ __ __ – __ __ __ __ UPIN or NPI #____________ |
|
|
|
SECTION B: Information in this Section May Not Be Completed by the Supplier of the Items/Supplies.
EST. LENGTH OF NEED (# OF MONTHS): ______
DIAGNOSIS CODES: ______ ______ ______ ______
ANSWERS |
QUESTIONS |
|
ANSWER QUESTIONS |
|
ANSWER QUESTIONS |
|
ANSWER QUESTIONS 6 AND 12 FOR ULTRASONIC OSTEOGENSIS STIMULATOR. |
|
(Check Y for Yes, N for No, or D for Does Not Apply. For questions about months, enter |
a) oY o N oD 6. In a fracture, has there been no clinically significant radiographic evidence of healing for a minimum of 90 days?
a)oY o N oD 7. (a) Does the patient have a failed fusion of a joint other than the spine?
(b)How many months prior to ordering the device did the patient have the fusion?
b )_____________
o Y o N o D |
8. Does the patient have a congenital pseudoarthrosis? |
a)oY o N oD 9. (a) Is the device being ordered as a treatment of a failed single level spinal fusion surgery in a patient who has not had a recent repeat fusion?
b) |
(b) How many months prior to ordering the device did the patient have the fusion? |
a)o Y o N o D 10. (a) Is the device being ordered as an adjunct to repeat single level spinal fusion surgery in a patient with a previously failed spinal fusion at the same level(s)?
b)_____________ |
(b) How many months prior to ordering the device did the patient have the repeat fusion? |
|
||
c)______________ |
(c) How many months prior to ordering the device did the patient have the previously failed fusion? |
|
||
|
|
|
|
|
o Y |
o |
N o D |
11. Is the device being ordered following multi¬level spinal fusion surgery? |
|
|
|
|
|
|
o Y o N o D |
12. Has there been at least one open surgical intervention for treatment of the fracture? |
|
||
NAME OF PERSON ANSWERING SECTION B QUESTIONS, IF OTHER THAN PHYSICIAN (Please Print):
NAME__________________________________ TITLE________________________EMPLOYER________________________
SECTION C: Narrative Description of Equipment and Cost
(1)Narrative description of Iall items, accessories and option ordered; (2) Suppliers charge; and (3) Medicare Fee Schedule Allowance for each item, accessory, and option (see instructions on back)
SECTION D: PHYSICIAN Attestation and Signature/Date
I certify that I am the treating physician identified in Section A of this form. I have received Sections A, B and C of the Certificate of Medical Necessity (including charges for items ordered). Any statement on my letterhead attached hereto, has been reviewed and signed by me. I certify that the medical necessity information in Section B is true, accurate and complete, to the best of my knowledge, and I understand that any falsification, omission, or concealment of material fact in that section may subject me to civil or criminal liability.
PHYSICIAN’S SIGNATURE_________________________________________________________DATE _____/_____/_____
Signature and Date Stamps Are Not Acceptable.
Form

INSTRUCTIONS FOR COMPLETING THE CERTIFICATE OF MEDICAL NECESSITY
FOR OSTEOGENESIS STIMULATORS
SECTION A: |
(May be completed by the supplier) |
CERTIFICATION |
If this is an initial certification for this patient, indicate this by placing date (MM/DD/YY) needed initially in the space TYPE/ |
DATE: |
marked “INITIAL.” If this is a revised certification (to be completed when the physician changes the order, based on the |
|
patient’s changing clinical needs), indicate the initial date needed in the space marked “INITIAL,” and indicate the |
|
recertification date in the space marked “REVISED.” If this is a recertification, indicate the initial date needed in the |
|
space marked “INITIAL,” and indicate the recertification date in the space marked “RECERTIFICATION.” Whether |
|
submitting a REVISED or a RECERTIFIED CMN, be sure to always furnish the INITIAL date as well as the REVISED or |
|
RECERTIFICATION date. |
PATIENT |
Indicate the patient’s name, permanent legal address, telephone number and his/her Medicare ID as it appears on his/her |
INFORMATION: |
Medicare card and on the claim form. |
SUPPLIER |
Indicate the name of your company (supplier name), address and telephone number along with the Medicare Supplier |
INFORMATION: |
Number assigned to you by the National Supplier Clearinghouse (NSC) or applicable National Provider Identifier (NPI). If |
|
using the NPI Number, indicate this by using the qualifier XX followed by the |
|
e.g. NSC number, use the qualifier 1C followed by the |
PLACE OF SERVICE: |
Indicate the place in which the item is being used, i.e., patient’s home is 12, skilled nursing facility (SNF) is 31, End |
|
Stage Renal Disease (ESRD) facility is 65, etc. Refer to the DMERC supplier manual for a complete list. |
FACILITY NAME: |
If the place of service is a facility, indicate the name and complete address of the facility. |
SUPPLY ITEM/SERVICE |
List all procedure codes for items ordered. Procedure codes that do not require certification should not be listed |
PROCEDURE CODE(S): |
on the CMN. |
PATIENT DOB, HEIGHT, |
Indicate patient’s date of birth (MM/DD/YY) and sex (male or female); height in inches and weight in pounds, if requested. |
WEIGHT AND SEX: |
|
PHYSICIAN NAME, |
Indicate the PHYSICIAN’S name and complete mailing address. |
ADDRESS: |
|
PHYSICIAN |
Accurately indicate the treating physician’s Unique Physician Identification Number (UPIN) or applicable National |
INFORMATION: |
Provider Identifier (NPI). If using the NPI Number, indicate this by using the qualifier XX followed by the |
|
If using UPIN number, use the qualifier 1G followed by the |
PHYSICIAN’S |
Indicate the telephone number where the physician can be contacted (preferably where records would be accessible |
TELEPHONE NO: |
pertaining to this patient) if more information is needed. |
SECTION B: |
(May not be completed by the supplier. While this section may be completed by a |
|
Physician employee, it must be reviewed, and the CMN signed (in Section D) by the treating practitioner.) |
EST. LENGTH OF NEED: |
Indicate the estimated length of need (the length of time the physician expects the patient to require use of the ordered |
|
item) by filling in the appropriate number of months. If the patient will require the item for the duration of his/her life, |
|
then enter “99”. |
DIAGNOSIS CODES: |
In the first space, list the diagnosis code that represents the primary reason for ordering this item. List any additional |
|
diagnosis codes that would further describe the medical need for the item (up to 4 codes). |
QUESTION SECTION: |
This section is used to gather clinical information to help Medicare determine the medical necessity for the item(s) |
|
being ordered. Answer each question which applies to the items ordered, checking “Y” for yes, “N” for no, or “D” for |
|
does not apply. |
NAME OF PERSON |
If a clinical professional other than the treating physician (e.g., home health nurse, physical therapist, dietician) or a |
ANSWERING SECTION B |
physician employee answers the questions of Section B, he/she must print his/her name, give his/her professional title |
QUESTIONS: |
and the name of his/her employer where indicated. If the physician is answering the questions, this space may be |
|
left blank. |
SECTION C: |
(To be completed by the supplier) |
NARRATIVE |
Supplier gives (1) a narrative description of the item(s) ordered, as well as all options, accessories, supplies and drugs; |
DESCRIPTION OF |
(2) the supplier’s charge for each item(s), options, accessories, supplies and drugs; and (3) the Medicare fee schedule |
EQUIPMENT & COST: |
allowance for each item(s), options, accessories, supplies and drugs, if applicable. |
SECTION D: |
(To be completed by the physician) |
PHYSICIAN |
The physician’s signature certifies (1) the CMN which he/she is reviewing includes Sections A, B, C and D; (2) the |
ATTESTATION: |
answers in Section B are correct; and (3) the |
PHYSICIAN SIGNATURE |
After completion and/or review by the physician of Sections A, B and C, the physician’s must sign and date the CMN in |
AND DATE: |
Section D, verifying the Attestation appearing in this Section. The physician’s signature also certifies the items ordered |
|
are medically necessary for this patient. |
According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is
DO NOT SUBMIT CLAIMS TO THIS ADDRESS. Please see http://www.medicare.gov/ for information on claim filing.
Form
Form Data
| Fact Name | Detail |
|---|---|
| Form Number | CMS-847 |
| Purpose | Certificate of Medical Necessity for Osteogenesis Stimulators |
| Approved by | Office of Management and Budget (OMB) |
| OMB Number | 0938-0679 |
| Expiration Date | 02/2024 |
| Governing Entity | Department of Health and Human Services, Centers for Medicare & Medicaid Services |
| Sections | A: Certification, Patient and Supplier Information; B: Medical Necessity Details; C: Equipment and Cost Description; D: Physician Attestation and Signature |
Instructions on Utilizing Cms 847
Filling out the CMS 847 form is an essential step in the process of documenting the medical necessity of osteogenesis stimulators for patients. This process ensures that patients receive the necessary treatments and that the provision of these treatments adheres to the guidelines set by Medicare. The form is divided into several sections, each requiring specific information about the patient, the supplier, and the medical necessity of the prescribed equipment. Here are the steps needed to complete the CMS 847 form accurately:
- Under Section A: Certification Type/Date, select the appropriate certification type (Initial, Revised, Recertification) and enter the corresponding date (MM/DD/YYYY).
- In the Patient Information area, provide the patient's name, permanent legal address, telephone number, and Medicare ID as indicated on their Medicare card.
- For Supplier Information, enter your company name, address, and telephone number along with your Medicare Supplier Number (NSC or NPI #).
- Specify the Place of Service by noting the environment in which the item will be used (e.g., patient’s home, skilled nursing facility).
- If the service is provided in a facility, you must include the Facility Name and complete address.
- List all Supply Item/Service/Procedure Code(s) relevant to the osteogenesis stimulator being provided.
- Include the Patient's Date of Birth (DOB), Height (in inches), Weight (in pounds), and Sex.
- In the section for Physician Information, note the physician's name, address, telephone number, and their Unique Physician Identification Number (UPIN) or National Provider Identifier (NPI) if applicable.
- Section B: Should not be completed by the supplier. This section requires estimated length of need, diagnosis codes, and answers to questions regarding the medical necessity of the device. This part must be completed by a clinician or patient care team member familiar with the patient’s medical history, and reviewed by the ordering physician.
- The person completing Section B should include their name, title, and employer if they are not the physician.
- In Section C: As the supplier, provide a narrative description of all items, accessories, and options ordered, the supplier’s charge for each, and the Medicare Fee Schedule Allowance, if applicable.
- Section D requires the physician's attestation and signature with date, confirming the medical necessity of the ordered items. Signature and date stamps are not acceptable.
Once the form is thoroughly filled out, ensure that all provided information is accurate and complete. This document will serve as a crucial piece of evidence supporting the medical necessity of the osteogenesis stimulator, enabling the patient to access the required treatment promptly. The accurate and conscientious completion of the CMS 847 form plays a pivotal role in this process.
Obtain Answers on Cms 847
What is the CMS-847 form?
The CMS-847 form is a Certificate of Medical Necessity for Osteogenesis Stimulators, approved by the Department of Health and Human Services and used by the Centers for Medicare & Medicaid Services (CMS). This document is essential for Medicare coverage, as it certifies the medical necessity of osteogenesis stimulators for a patient. The form outlines the patient's details, diagnosis codes, the physician's attestation, and a detailed description of the equipment and its cost.
Who completes the CMS-847 form?
Section A can be completed by the supplier of the osteogenesis stimulator, providing details about the certification type and date, patient information, supplier name, and the place of service. Section B, containing the clinical need and diagnosis codes, should be completed by someone other than the supplier, such as a clinician or physician employee, but must be reviewed and signed by the treating physician. Section C is for the supplier to describe the equipment and cost, and finally, Section D must be filled out by the treating physician, certifying the necessity of the prescribed item.
When is the CMS-847 form required?
The form is required when osteogenesis stimulators are prescribed as medically necessary for a patient's treatment. It needs to be completed and submitted to ensure Medicare coverage for these devices. This includes scenarios such as initial certification, revised certification due to changes in clinical needs, or recertification to continue the use of the equipment.
How is the estimated length of need determined on the CMS-847 form?
In the estimated length of need section, the treating physician will indicate the expected duration the patient will require the osteogenesis stimulator. This is expressed in months, with options ranging from 1 to 99, where “99” represents a lifetime need. This estimate is based on the physician's clinical judgment and understanding of the patient's specific health condition and recovery expectations.
Can the CMS-847 form be signed with a signature stamp?
No, the use of signature stamps for the physician’s signature in Section D is explicitly not acceptable. The form requires a handwritten signature by the treating physician to certify the information provided in the form, ensuring that the items ordered are medically necessary for the patient. This direct signature adds a layer of verification and accountability to the process.
Where can I find the CMS-847 form and how do I submit it?
The CMS-847 form can be accessed through the official Centers for Medicare & Medicaid Services website or by contacting a Medicare office. Once completed, the form should be submitted following the guidelines provided by Medicare for durable medical equipment coverage. Submission details, including where to send the form, can vary, so it’s important to check with Medicare or a professional healthcare provider to ensure proper processing.
Common mistakes
Completing the CMS-847 form, a Certificate of Medical Necessity for Osteogenesis Stimulators, is a crucial step in securing coverage for essential medical equipment. However, errors can delay this process, impacting the timely provision of care. Below, common mistakes made when filling out this form are highlighted to help ensure accuracy and completeness.
- Incorrect or Incomplete Patient Information: Failing to provide the complete patient name, address, telephone, and Medicare ID as it appears on the Medicare card and claim form can lead to processing delays.
- Supplier Information Errors: Not including the accurate supplier name, address, telephone number, and either the NSC or NPI number can lead to miscommunication or misattribution of equipment provision.
- Place of Service Omissions: Neglecting to specify the place where the item is being used, for instance, a patient’s home or a skilled nursing facility, with the correct code can result in claim denial.
- Procedure Codes Misalignment: Listing incorrect or unrelated procedure codes for items ordered or leaving this section incomplete can misguide the certification process.
- Physician Information Accuracy: Not accurately providing the treating physician’s name, address, telephone number, and either the UPIN or NPI number complicates verification processes.
- Estimation of Need Discrepancies: Incorrectly estimating the length of need or number of months the patient requires the equipment can affect coverage scope.
- Diagnosis Codes Confusion: Misrepresenting or omitting the primary diagnosis code and any relevant additional diagnosis codes that justify medical necessity can hinder approval.
- Unanswered or Incorrectly Answered Questions: Failing to answer applicable questions or providing incorrect answers in Section B, which is essential for establishing the medical necessity of the device.
- Physician Attestation Omissions: The lack of a physician’s signature and/or date in Section D, confirming the medical necessity of the items requested, poses a significant barrier to claim processing.
Addressing these common mistakes with precision and care can facilitate smoother processing of the CMS-847 form, ultimately supporting patient access to medically necessary osteogenesis stimulators.
Documents used along the form
In the realm of healthcare and medical services, particularly those revolving around osteogenesis stimulators, the Certificate of Medical Necessity (CMS-847) form stands out as a critical document. However, in order to ensure comprehensive coverage and compliance, several other forms and documents often accompany the CMS-847 form in submissions. These additional documents play pivotal roles in establishing the medical necessity, providing detailed patient information, and ensuring that all regulatory and insurance requirements are met.
- CMS-855S: This application form is for suppliers of durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS). It is necessary for suppliers to be registered and recognized by Medicare to provide and bill for these services.
- HCFA-1500: A standard claim form used by physicians and many other healthcare providers for claim billing. Although electronic submission is increasingly common, this form may still be used for paper submissions. It captures patient information, services provided, and billing details.
- Proof of Delivery Documentation: This documentation is essential to verify the delivery of DMEPOS items to the patient, detailing what was delivered, the quantity, and when it was received.
- Detailed Written Order (DWO): Before billing Medicare, a supplier must have a DWO from the treating physician, detailing the necessary equipment, including any accessories and supplies.
- Advance Beneficiary Notice of Noncoverage (ABN): This form is used to notify patients that Medicare may not cover a specific service, allowing them to make informed decisions about receiving and paying for services that might not be reimbursed.
- Medical Records: Patient medical records supporting the medical necessity of the DMEPOS item prescribed are often required. These should include relevant medical history, examinations, test results, and treatments.
- Physician Progress Notes: Notes that document the patient's condition over time, showing the progression of the ailment and the need for the prescribed DMEPOS item.
- Letter of Medical Necessity (LMN): While the CMS-847 serves a similar purpose, an LMN provides detailed reasoning from the physician on why the prescribed item is necessary for the patient's condition.
- Appeal Documentation: If a claim is initially denied, detailed appeal letters along with additional supporting documentation may be necessary to contest the decision.
- Privacy Release Forms: Given the sensitive nature of health information, patients must consent to the sharing of their medical records between providers, suppliers, and insurers.
Collectively, these documents ensure a holistic approach to patient care, regulatory adherence, and the facilitation of billing and claims processes. It is crucial for healthcare providers and suppliers to be cognizant of the comprehensive documentation requirements to prevent delays or denials in coverage. Understanding each document's role enhances the efficacy of healthcare delivery and ensures compliance with Medicare guidelines.
Similar forms
The CMS-855A form, which is the Medicare Enrollment Application for Institutional Providers, shares similarities with the CMS-847 form in its purpose of ensuring compliance and verifying the need and eligibility for services. Both forms require detailed provider and service information, including Medicare identification numbers and certification of the information provided.
The CMS-1500, the standard claim form used by healthcare practitioners and suppliers to bill Medicare Part B services, is akin to the CMS-847 in that it also necessitates detailed patient information, diagnosis codes, and service codes. Both documents serve as critical elements in the Medicare billing process, ensuring that the services provided are accurately recorded and billed.
The Home Health Certification and Plan of Care (CMS-485) also resembles the CMS-847 form. This document is necessary for home health services, requiring a physician’s certification of the patient’s eligibility for home health benefits, much like the CMS-847 requires a physician’s certification of medical necessity for osteogenesis stimulators.
The Health Insurance Benefit Agreement (CMS-1561), which must be completed by institutions wishing to participate in the Medicare program, is comparable to the CMS-847. Both forms are part of the broader Medicare documentation process, designed to verify the provision and medical necessity of services to Medicare beneficiaries.
Last, the Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) Supplier Standards (CMS-10036) bears resemblance to the CMS-847, as both documents involve durable medical equipment. The CMS-10036 outlines the standards suppliers must meet, while the CMS-847 certifies the medical necessity of specific DME items for patients, underscoring the diverse documentation involved in DME provision and coverage.
Dos and Don'ts
When filling out the CMS 847 form for osteogenesis stimulators, it is important to follow specific guidelines to ensure the form is completed accurately and efficiently. Below are six dos and don'ts to consider:
- Do ensure that all information provided in Section A regarding certification type and date, patient and supplier details, and place of service is complete and accurate.
- Do carefully read the instructions before starting the form to avoid any mistakes in filling out the required fields.
- Do answer all relevant questions in Section B regarding the patient's diagnosis, the estimated length of need, and specific details relating to the type of osteogenesis stimulator required, ensuring that these answers are based on the patient's medical records and the physician's assessment.
- Don't let the supplier of the items/supplies complete Section B, as this section may not be completed by the supplier and must be based on the treating practitioner's medical evaluation of the patient.
- Don't leave any applicable fields blank. For example, if certain questions in Section B do not apply to your patient's situation, ensure to mark them as "Does Not Apply" or "D" instead of skipping them.
- Don't forget to obtain the physician's signature and date in Section D, as this is a critical part of the certification process. The physician must certify they have reviewed Sections A, B, and C and that all information is true and accurate to the best of their knowledge.
Following these guidelines can help facilitate a smoother process in submitting the CMS 847 form and supporting the patient's need for an osteogenesis stimulator.
Misconceptions
There are several misconceptions about the CMS-847 form, a Certificate of Medical Necessity for Osteogenesis Stimulators. Understanding these misconceptions is essential for clear communication and efficient processing. Below are seven common misunderstandings:
- Misconception 1: The CMS-847 form is only for spinal conditions. While the form does include sections specifically for spinal electrical osteogenesis stimulators, it also covers nonspinal electrical and ultrasonic osteogenesis stimulators. This diversity addresses various conditions needing osteogenesis stimulation.
- Misconception 2: Any healthcare provider can complete the entire form. In reality, Section B of the form, which collects detailed patient and medical necessity information, may not be completed by the supplier. Instead, it must be filled out by a clinical professional or the treating physician, who must also sign Section D to attest to the medical necessity of the ordered item(s).
- Misconception 3: Suppliers can fill out Section C only. Suppliers are actually responsible for completing both Section A, which includes patient and supplier information, and Section C, which describes the equipment, cost, and Medicare fee schedule allowance.
- Misconception 4: The form serves as an immediate approval for Medicare coverage. Completion and submission of the CMS-847 form start the process for determining coverage but do not guarantee it. Medicare uses the information provided to assess medical necessity and eligibility for coverage.
- Misconception 5: The CMS-847 form is valid indefinitely. The form comes with an expiration date, clearly stated at the top. As of the current version, it expires in 02/2024. It's important to use the most current form to ensure compliance with Medicare requirements.
- Misconception 6: Physician’s signature can be a stamp. According to the instructions, signature and date stamps are not acceptable for the physician’s attestation in Section D. The physician must provide an original signature to verify the form, ensuring that the patient information and medical necessity details have been accurately and personally reviewed.
- Misconception 7: The CMS-847 is complicated and time-consuming. While the form is detailed, its structure is straightforward. With clear instructions for each section, it is designed for efficient completion. The estimated time for filling it out is an average of 12 minutes, which facilitates prompt submission.
Addressing these misconceptions is pivotal in streamlining the process for obtaining osteogenesis stimulators through Medicare, ensuring patients receive the necessary equipment without undue delay.
Key takeaways
Filling out the CMS-847 form is a crucial step for patients who require osteogenesis stimulators, as it helps in establishing the medical necessity for Medicare coverage. Here are key takeaways to ensure accuracy and compliance during this process:
- Ensure that all sections of the form are completed accurately, with the initial step being the certification type and date, which distinguishes between initial certification, revised certification, and recertification.
- Section A requires basic patient and supplier information, including Medicare ID and NSC or NPI number. It's vital to double-check these details for accuracy to avoid processing delays.
- The place of service, indicated in the form, should reflect the correct location where the device will be used, as this can affect Medicare coverage eligibility.
- In Section B, the emphasis is placed on the medical necessity of the device. This section cannot be filled out by the supplier but can be completed by a non-physician clinician or physician employee and must be reviewed and signed by the treating physician.
- Questions 6 through 12 in Section B are designed to specifically ascertain the need for the osteogenesis stimulator based on the patient's medical condition and history, ensuring that the device is medically necessary.
- Section C, which is to be filled out by the supplier, requires a detailed narrative description of the equipment, including all items, accessories, and options ordered, along with the supplier's charge and the Medicare fee schedule allowance for each.
- The physician’s attestation and signature in Section D certifies the medical necessity of the items ordered and confirms the accuracy of the information provided in the form.
- Understanding the instructions for completing the CMS-847 form is essential for both suppliers and healthcare professionals to ensure compliance with Medicare requirements and to facilitate the timely approval and provision of osteogenesis stimulators for patients in need.
By adhering to these key points, healthcare providers and suppliers can navigate the complexities of Medicare documentation, ensuring that patients receive the necessary medical equipment in a timely and efficient manner.
Popular PDF Forms
Llc 800 Payment Due Date - Businesses filing this form initiate their journey within California's legal and regulatory business environment.
Cf 1R Alt Hvac - Details insulation requirements for newly installed or replaced ducts as per the energy efficiency standards for different climate zones.