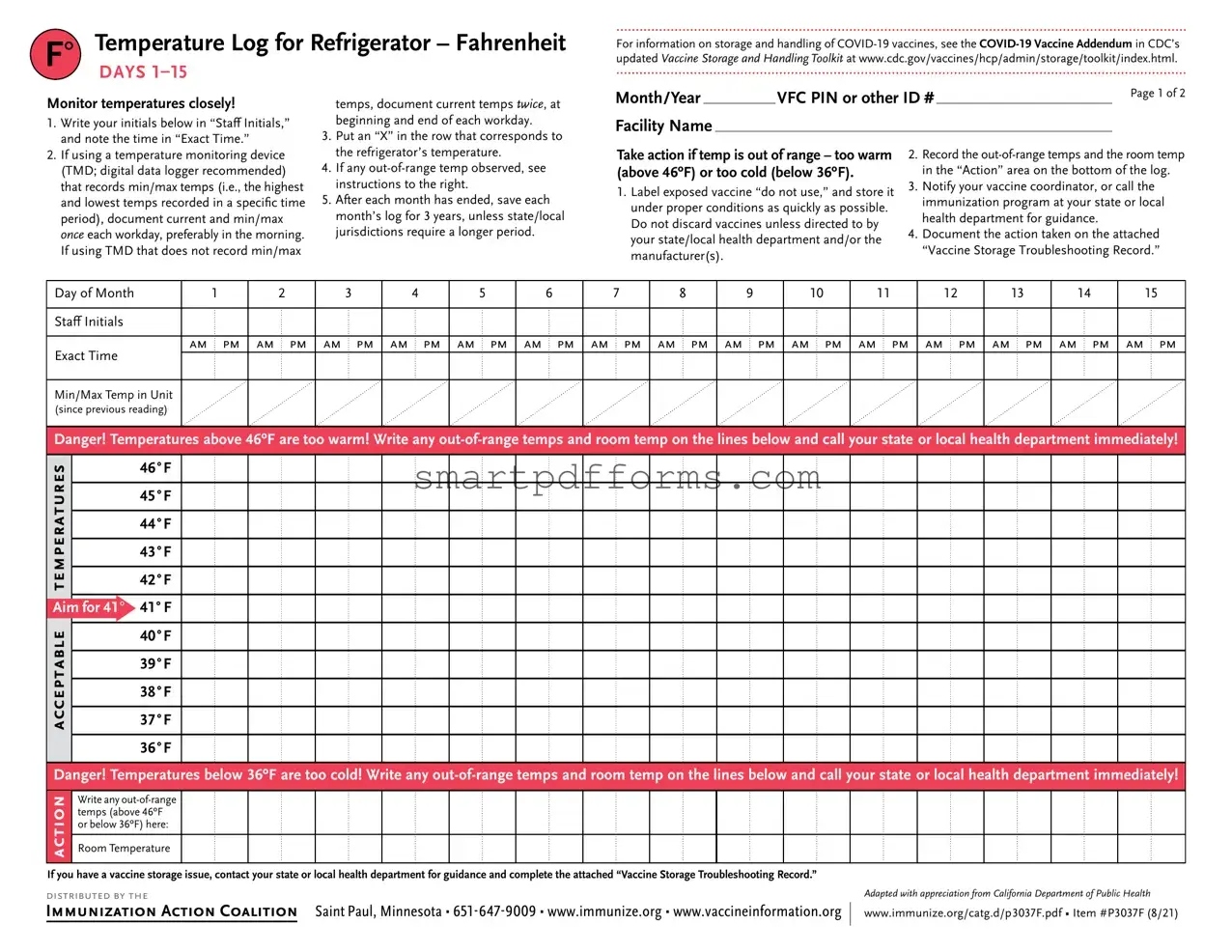
Blank Refrigerator Temperature Log PDF Template
In the realm of healthcare and immunization, the meticulous documentation and monitoring of vaccine storage temperatures are critical. The Refrigerator Temperature Log form plays a pivotal role in this process, providing a structured means for tracking the temperatures within refrigeration units to ensure they remain within the specified safe range. This form, essential for the storage of COVID-19 vaccines among others, delineates a straightforward protocol for daily temperature recording, including the documentation of minimum and maximum temperatures, and specifies clear steps to be taken in the event of a temperature excursion. Moreover, it emphasizes the importance of timely corrective action and maintaining logs for a minimum of three years, or longer if mandated by state or local jurisdictions. Outlined are instructions for notating staff initials, exact timing of temperature checks, and direct guidance on actions required should temperatures fall outside the safe threshold—either too warm (above 46ºF) or too cold (below 36ºF). This document also incorporates a Vaccine Storage Troubleshooting Record, underscoring the necessary steps to document any storage anomalies and the subsequent actions to mitigate any potential impact on vaccine efficacy. Distributed by the Immunization Action Coalition, this log form and accompanying instructions enrich the toolkit provided by the CDC, aiming to preserve the integrity of vaccines through rigorous monitoring and expedient responses to any signs of storage compromise.
Preview - Refrigerator Temperature Log Form
F° |
Temperature Log for Refrigerator – Fahrenheit |
DAYS |
For information on storage and handling of
Monitor temperatures closely!
temps, document current temps twice, at
Month/Year |
|
VFC PIN or other ID # |
|
Page 1 of 2 |
|
|
|
1.Write your initials below in “Staff Initials,” and note the time in “Exact Time.”
2.If using a temperature monitoring device
(TMD; digital data logger recommended) that records min/max temps (i.e., the highest and lowest temps recorded in a specific time period), document current and min/max once each workday, preferably in the morning. If using TMD that does not record min/max
beginning and end of each workday.
3.Put an “X” in the row that corresponds to the refrigerator’s temperature.
4.If any
5.After each month has ended, save each month’s log for 3 years, unless state/local jurisdictions require a longer period.
Facility Name
Take action if temp is out of range – too warm (above 46ºF) or too cold (below 36ºF).
1.Label exposed vaccine “do not use,” and store it under proper conditions as quickly as possible. Do not discard vaccines unless directed to by your state/local health department and/or the manufacturer(s).
2.Record the
3.Notify your vaccine coordinator, or call the immunization program at your state or local health department for guidance.
4.Document the action taken on the attached
“Vaccine Storage Troubleshooting Record.”
Day of Month |
1 |
|
2 |
|
3 |
|
4 |
|
5 |
|
6 |
|
7 |
|
8 |
|
9 |
|
10 |
|
11 |
|
12 |
|
13 |
|
14 |
|
15 |
Staff Initials |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
Exact Time |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Min/Max Temp in Unit |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(since previous reading) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Danger! Temperatures above 46ºF are too warm! Write any
temperatures
Aim for 41º
acceptable
46˚F
45˚F
44˚F
43˚F
42˚F
41˚F
40˚F
39˚F
38˚F
37˚F
36˚F
Danger! Temperatures below 36ºF are too cold! Write any
action
Write any
Room Temperature
If you have a vaccine storage issue, contact your state or local health department for guidance and complete the attached “Vaccine Storage Troubleshooting Record.”
DISTRIBUTED BY THE
IMMUNIZATION ACTION COALITION Saint Paul, Minnesota •
Adapted with appreciation from California Department of Public Health
www.immunize.org/catg.d/p3037F.pdf • Item #P3037F (8/21)
F° |
Temperature Log for Refrigerator – Fahrenheit |
DAYS 16 |
For information on storage and handling of
Monitor temperatures closely!
temps, document current temps twice, at
Month/Year |
|
VFC PIN or other ID # |
|
Page 2 of 2 |
1.Write your initials below in “Staff Initials,” and note the time in “Exact Time.”
2.If using a temperature monitoring device
(TMD; digital data logger recommended) that records min/max temps (i.e., the highest and lowest temps recorded in a specific time period), document current and min/max once each workday, preferably in the morning. If using TMD that does not record min/max
beginning and end of each workday.
3.Put an “X” in the row that corresponds to the refrigerator’s temperature.
4.If any
5.After each month has ended, save each month’s log for 3 years, unless state/local jurisdictions require a longer period.
Facility Name
Take action if temp is out of range – too warm (above 46ºF) or too cold (below 36ºF).
1.Label exposed vaccine “do not use,” and store it under proper conditions as quickly as possible. Do not discard vaccines unless directed to by your state/local health department and/or the manufacturer(s).
2.Record the
3.Notify your vaccine coordinator, or call the immunization program at your state or local health department for guidance.
4.Document the action taken on the attached
“Vaccine Storage Troubleshooting Record.”
Day of Month |
16 |
|
17 |
|
18 |
|
19 |
|
20 |
|
21 |
|
22 |
|
23 |
|
24 |
|
25 |
|
26 |
|
27 |
|
28 |
|
29 |
|
30 |
|
31 |
Staff Initials |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
am |
pm |
Exact Time |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Min/Max Temp in Unit |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(since previous reading) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Danger! Temperatures above 46ºF are too warm! Write any
temperatures
Aim for 41º
acceptable
46˚F
45˚F
44˚F
43˚F
42˚F
41˚F
40˚F
39˚F
38˚F
37˚F
36˚F
Danger! Temperatures below 36ºF are too cold! Write any
action
Write any
Room Temperature
If you have a vaccine storage issue, contact your state or local health department for guidance and complete the attached “Vaccine Storage Troubleshooting Record.”
DISTRIBUTED BY THE
IMMUNIZATION ACTION COALITION Saint Paul, Minnesota •
Adapted with appreciation from California Department of Public Health
www.immunize.org/catg.d/p3037F.pdf • Item #P3037F (8/21)

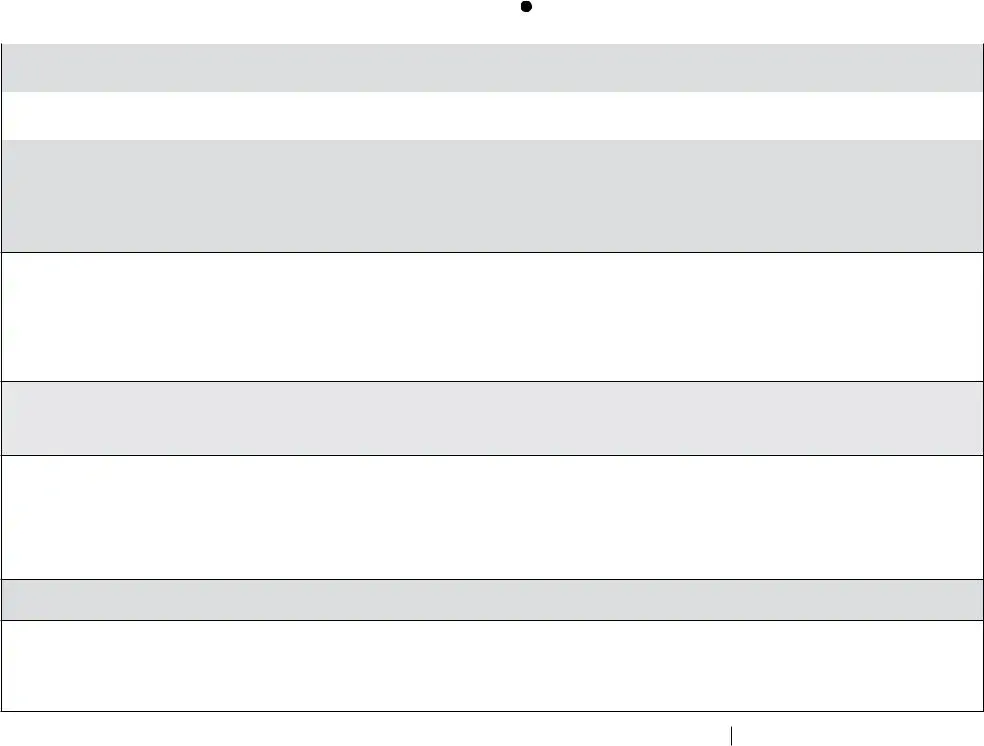
Vaccine Storage Troubleshooting Record
(check one) □Refrigerator □Freezer
Use this form to document any unacceptable vaccine storage event, such as exposure of refrigerated vaccines to temperatures that are outside the manufacturers’ recommended storage ranges.
A fillable pdf of this form is available at www.immunize. org/catg.d/p3041.pdf
Date & Time of Event |
Storage Unit Temperature |
Room Temperature |
Person Completing Report |
|
|
If multiple, related events occurred, |
at the time the problem was discovered |
at the time the problem was discovered |
|
|
|
see Description of Event below. |
|
|
|
|
|
|
|
|
|
|
|
Date: |
Temp when discovered: |
|
Temp when discovered: |
Name: |
|
|
|
|
|
|
|
Time: |
Minimum temp: |
Maximum temp: |
Comment (optional): |
Title: |
Date: |
|
|
|
|
|
|
Description of Event (If multiple, related events occurred, list each date, time, and length of time out of storage.)
•General description (i.e., what happened?)
•Estimated length of time between event and last documented reading of storage temperature in acceptable range (2o to 8oC [36o to 46oF] for refrigerator;
• Inventory of affected vaccines, including (1) lot #s and (2) whether purchased with public (for example, VFC) or private funds (Use separate sheet if needed, but maintain the inventory with this troubleshooting record.)
•At the time of the event, what else was in the storage unit? For example, were there water bottles in the refrigerator and/or frozen coolant packs in the freezer?
•Prior to this event, have there been any storage problems with this unit and/or with the affected vaccine?
•Include any other information you feel might be relevant to understanding the event.
Action Taken (Document thoroughly. This information is critical to determining whether the vaccine might still be viable!)
•When were the affected vaccines placed in proper storage conditions? (Note: Do not discard the vaccine. Store exposed vaccine in proper conditions and label it “do not use” until after you can discuss with your state/ local health department and/or the manufacturer[s].)
•Who was contacted regarding the incident? (For example, supervisor, state/local health department,
•IMPORTANT: What did you do to prevent a similar problem from occurring in the future?
Results
• What happened to the vaccine? Was it able to be used? If not, was it returned to the distributor? (Note: For
DISTRIBUTED BY THE
IMMUNIZATION ACTION COALITION Saint Paul, Minnesota •
www.immunize.org/catg.d/p3041.pdf • Item #P3041 (8/21)
Vaccine Storage Troubleshooting Record (check one) |
◯ |
◯ |
◯ |
Refrigerator |
Freezer |
Use this form to document any unacceptable vaccine storage event, such as exposure of refrigerated vaccines to temperatures that are outside the manufacturers' recommended storage ranges.
Date & Time of Event |
Storage Unit Temperature |
Room Temperature |
Person Completing Report |
|
|
If multiple, related events occurred, |
at the time the problem was discovered |
at the time the problem was discovered |
|
|
|
see Description of Event below. |
|
|
|
|
|
|
|
|
|
|
|
Date: (see below) |
Temp when discovered: |
45º F |
Temp when discovered: 77º F |
Name: Natalie Nurse |
|
|
|
|
|
|
|
Time: (see below) |
Minimum temp: 38º F |
Maximum temp: 53º F |
Comment (optional):temp is approx. |
Title: VFC Coordinator |
Date: 6/29/21 |
Description of Event (If multiple, related events occurred, list each date, time, and length of time out of storage.)
•General description (i.e., what happened?)
•Estimated length of time between event and last documented reading of storage temperature in acceptable range (2o to 8oC [36o to 46oF] for refrigerator;
• Inventory of affected vaccines, including (1) lot #s and (2) whether purchased with public (for example, VFC) or private funds (Use separate sheet if needed, but maintain the inventory with this troubleshooting record.)
•At the time of the event, what else was in the storage unit? For example, were there water bottles in the refrigerator and/or frozen coolant packs in the freezer?
•Prior to this event, have there been any storage problems with this unit and/or with the affected vaccine?
•Include any other information you feel might be relevant to understanding the event.
At 8 am on Tuesday (6/29/21) morning when clinic opened, identified 4 temperature excursions over the weekend in refrigerator with readings as high as 54°, 50°, 49° & 53°F in primary vaccine storage unit #1. Recordings taken every 15 min on calibrated digital data logger overnight. Data logger probe in glycol located in middle of refrigerator with vaccines.
Total time out of range: approximately 3 hrs — maximum temp 53°F (see attached document of continuous temp readings)
Inventory of vaccines: see attached
Water bottles in refrigerator door. No vaccine stored in freezer. No problems with storage unit prior to Saturday night. Thunderstorms in area over weekend may have affected power.
Action Taken (Document thoroughly. This information is critical to determining whether the vaccine might still be viable!)
•When were the affected vaccines placed in proper storage conditions? (Note: Do not discard the vaccine. Store exposed vaccine in proper conditions and label it “do not use” until after you can discuss with your state/ local health department and/or the manufacturer[s].)
•Who was contacted regarding the incident? (For example, supervisor, state/local health department,
•IMPORTANT: What did you do to prevent a similar problem from occurring in the future?
Vaccines currently stored appropriately at 41ºF. Refrigerator and vaccines labeled "Do Not Use."
My State Immunization Program contacted at 8:30 am. Spoke with Victor Vaccine. Provided Victor with details of event and list of vaccines. Vaccine to remain quarantined until we hear back from Victor.
Called electric company and confirmed 2 short power outages during weekend. Checked refrigerator seals called refrigerator maintenance company to replace seals.
Checked plug on unit placed tape over plug to prevent inadvertent dislodging. Plan to purchase plug guard.
Plan to follow up with Immunization Program on data loggers with alarms that could be sent to coordinator and
Results
• What happened to the vaccine? Was it able to be used? If not, was it returned to the distributor? (Note: For
Late on Monday, I talked with Victor regarding continued use of vaccine. Victor had checked with manufacturers which confirmed that vaccine is acceptable for use. He told me that vaccine could therefore be removed from quarantine. I discussed the entire situation with Susie Supervisor and Dr. Director (clinic medical director) who agreed that we could put vaccine back in use.
DISTRIBUTED BY THE
IMMUNIZATION ACTION COALITION Saint Paul, Minnesota •
www.immunize.org/catg.d/p3041.pdf • Item #P3041 (8/21)

Vaccine Storage Troubleshooting Record (check one) |
◯ |
◯ |
◯ |
Refrigerator |
Freezer |
Use this form to document any unacceptable vaccine storage event, such as exposure of refrigerated vaccines to temperatures that are outside the manufacturers' recommended storage ranges.
Date & Time of Event |
Storage Unit Temperature |
Room Temperature |
Person Completing Report |
|
|
If multiple, related events occurred, |
at the time the problem was discovered |
at the time the problem was discovered |
|
|
|
see Description of Event below. |
|
|
|
|
|
|
|
|
|
|
|
Date:7/13/2021 |
Temp when discovered: |
28º F |
Temp when discovered: 77º F |
Name: Natalie Nurse |
|
|
|
|
|
|
|
Time: 8:00 am |
Minimum temp: 28º F |
Maximum temp: 42º F |
Comment (optional):temp is approx. |
Title: VFC Coordinator |
Date: 7/13/21 |
Description of Event (If multiple, related events occurred, list each date, time, and length of time out of storage.)
•General description (i.e., what happened?)
•Estimated length of time between event and last documented reading of storage temperature in acceptable range (2o to 8oC [36o to 46oF] for refrigerator;
• Inventory of affected vaccines, including (1) lot #s and (2) whether purchased with public (for example, VFC) or private funds (Use separate sheet if needed, but maintain the inventory with this troubleshooting record.)
•At the time of the event, what else was in the storage unit? For example, were there water bottles in the refrigerator and/or frozen coolant packs in the freezer?
•Prior to this event, have there been any storage problems with this unit and/or with the affected vaccine?
•Include any other information you feel might be relevant to understanding the event.
When checked main clinic fridge (in lab) at 8:00 am on Tuesday, 7/13/2021, digital readout on data logger read 28ºF. Data logger located in center of fridge with probe in glycol . Review of computer readings (taken every 15 minutes) showed steady drop in temps from 42ºF at 8:15 pm (7/12/2021) to 28ºF reading discovered when arrived at clinic on Tuesday morning (7/13/2021). Readings hit 34ºF at 11 pm (7/12) and 32ºF at 2 am (7/13). Total time out of recommended storage temps = 9 hours, with 6 hours at freezing or below (see attached document of continuous temp readings). Inventory of vaccines attached.
Water bottles in refrigerator door and crisper area. No vaccines stored in freezer. No recent adjustments to temp controls and no previous temp excursions noted with this refrigerator before 7/13.
Action Taken (Document thoroughly. This information is critical to determining whether the vaccine might still be viable!)
•When were the affected vaccines placed in proper storage conditions? (Note: Do not discard the vaccine. Store exposed vaccine in proper conditions and label it “do not use” until after you can discuss with your state/ local health department and/or the manufacturer[s].)
•Who was contacted regarding the incident? (For example, supervisor, state/local health department,
•IMPORTANT: What did you do to prevent a similar problem from occurring in the future?
Upon discovery, vaccines marked “Do Not Use” and stored in 2nd clinic fridge (in exam room #3 at 41ºF). Also placed “Do Not Use” note on main fridge in lab. Notified Susie Supervisor about the issue. Contacted Victor Vaccine at My State Immunization Program at 8:30 am. Provided Victor with details of event and list of vaccines in fridge. Victor said to maintain vaccines in 2nd fridge and that he would check with manufacturers to determine next steps.
Called Jim’s Appliance Repair to examine fridge. Repairman found and replaced faulty thermostat in unit. Reset data logger on center shelf in fridge with probe in glycol .
Results
• What happened to the vaccine? Was it able to be used? If not, was it returned to the distributor? (Note: For
After fridge thermostat repaired, monitored temps in empty fridge for 1 week, per state requirements. Fridge maintained
DISTRIBUTED BY THE
IMMUNIZATION ACTION COALITION Saint Paul, Minnesota •
www.immunize.org/catg.d/p3041.pdf • Item #P3041 (8/21)
Form Data
| Fact Name | Description |
|---|---|
| Content Overview | The form logs the Fahrenheit temperatures of a refrigerator for the storage of vaccines, covering days 1–31, with additional guidelines for handling COVID-19 vaccines. |
| Temperature Monitoring | It requires documenting the current, minimum, and maximum temperatures twice daily, ideally at the beginning and the end of each workday. |
| Out-of-Range Protocol | Instructions are provided for actions to take if the refrigerator's temperature falls outside the acceptable range (below 36ºF or above 46ºF), including labeling vaccines "do not use," recording temperatures, and notifying relevant authorities. |
| Retention Period | Each month's log must be saved for 3 years, unless state or local jurisdictions require a longer period. |
| Troubleshooting Record | An attached "Vaccine Storage Troubleshooting Record" is included for documenting any instances where vaccines are exposed to temperatures outside recommended storage ranges. |
| Distribution | The form and its addendum are distributed by the Immunization Action Coalition, based in Saint Paul, Minnesota. |
| Source Acknowledgment | It is adapted with appreciation from the California Department of Public Health. |
| Additional Resources | Links to the CDC's Vaccine Storage and Handling Toolkit and a fillable PDF version of the Troubleshooting Record are provided for comprehensive guidelines and assistance. |
Instructions on Utilizing Refrigerator Temperature Log
Filling out the Refrigerator Temperature Log form is a crucial task that ensures the vaccines stored within the refrigerator are kept at safe temperatures. This action is not only a compliance measure but also a best practice to guarantee the effectiveness of the vaccines. Here's a step-by-step guide on how to accurately complete this form:
- Enter your facility name at the top of the form to identify which location this temperature log belongs to.
- In the “Month/Year” field, write down the current month and year for which you are tracking the refrigerator's temperatures.
- Fill in the “VFC PIN or other ID #” to provide a unique identifier associated with your facility or vaccine program.
- For each day of the month, documented under the "Day of Month" section, two readings are required:
- In the “Staff Initials” section, write your initials to note who is responsible for recording the temperatures.
- Note the “Exact Time” of the readings—one in the AM and one in the PM.
- Document the “Min/Max Temp in Unit (since previous reading)” if your Temperature Monitoring Device (TMD) records minimum and maximum temperatures. If your TMD does not record min/max temperatures, just record the current temperature twice daily.
- Place an “X” in the row that matches the refrigerator’s exact temperature for each time you check.
- If a temperature is out of the recommended range (below 36ºF or above 46ºF), follow the actions outlined on the form for out-of-range temperatures, documenting each step taken in the “Action” area of the form.
- At the bottom of the form, under “Room Temperature,” note the current room temperature when you log the refrigerator's temperature.
- Upon noticing a temperature that is out of range, record it in the provided space and immediately call your state or local health department for guidance.
- For more severe incidents or issues with vaccine storage, complete the attached “Vaccine Storage Troubleshooting Record,” detailing the event, actions taken, and final outcomes. This section is crucial for documenting any issues that could affect vaccine viability.
- Save each completed log for three years, or longer if required by your state or local jurisdiction.
Accurately filling out and diligently following the Refrigerator Temperature Log form are vital to maintaining vaccines' potency. By keeping a detailed record, healthcare facilities can swiftly address any issues that might compromise vaccine storage conditions, ensuring those needing vaccinations receive effective doses.
Obtain Answers on Refrigerator Temperature Log
What is the purpose of the Refrigerator Temperature Log form?
The Refrigerator Temperature Log form is designed to monitor and document temperatures within a refrigerator, ensuring they stay within safe limits, particularly for the storage of COVID-19 vaccines. This documentation is critical for maintaining vaccine efficacy and safety.How often should temperatures be recorded on the log?
Current temperatures should be documented twice each day. For those using a temperature monitoring device (TMD) that records minimum and maximum temperatures, these should be documented once each workday, preferably in the morning.What should be done if a temperature reading is out of the recommended range?
If temperatures are recorded above 46ºF or below 36ºF, staff should label exposed vaccines with “do not use,” store them under proper conditions as swiftly as possible, document the out-of-range temperatures and the room temperature in the “Action” area at the bottom of the log, notify the vaccine coordinator or the local health department for guidance, and document the action taken on the attached “Vaccine Storage Troubleshooting Record.”How long should completed Refrigerator Temperature Logs be retained?
Each month’s log should be saved for 3 years, unless there are state or local jurisdictions requirements for a longer period.What actions should be taken if vaccines are exposed to out-of-range temperatures?
Vaccines exposed to temperatures outside the recommended range should be labeled “do not use,” placed in proper storage conditions quickly, and not discarded unless directed by your state/local health department and/or the manufacturer(s). The next steps include recording the incident, contacting your vaccine coordinator or local health department, and documenting the actions taken on the “Vaccine Storage Troubleshooting Record.”What is the acceptable temperature range for vaccine storage in the refrigerator?
The acceptable temperature range for storing vaccines in the refrigerator is between 36ºF and 46ºF.What should be included in the "action" area of the log?
In the “Action” area, document any temperatures that fall outside the acceptable range (above 46ºF or below 36ºF), the room temperature at that time, and any actions taken in response to the temperature excursion.Who should be notified if there is an issue with vaccine storage temperatures?
If there is an issue with the vaccine storage temperatures, notify your vaccine coordinator or contact the immunization program at your state or local health department for guidance.What should be done at the end of each month with the temperature log?
At the end of each month, ensure that the log is complete, review it for any actions that need to be taken or followed up on, and then save the log for the appropriate retention period of 3 years or longer if required by state or local regulations.Where can additional information about vaccine storage and handling be found?
For more information on vaccine storage and handling, including for COVID-19 vaccines, visit the CDC’s Vaccine Storage and Handling Toolkit online at www.cdc.gov/vaccines/hcp/admin/storage/toolkit/index.html.
Common mistakes
When people are filling out the Refrigerator Temperature Log form for monitoring vaccine storage, several common mistakes can occur. Avoiding these errors ensures vaccines are stored under optimal conditions, which is critical for their effectiveness. Here are four common mistakes:
-
Not recording temperatures at the correct times: The form requires documenting temperatures twice each day, in the morning and in the evening. People often forget to record one of these crucial measurements, which can lead to missing out on detecting temperature deviations in a timely manner.
-
Failing to document minimum and maximum temperatures: For those using a Temperature Monitoring Device (TMD) that records the highest and lowest temperatures, it's important to document these ranges daily. This oversight can result in not capturing extreme temperature fluctuations that might compromise the vaccines.
-
Incorrectly marking the temperature on the log: The form includes a specific section for marking the current refrigerator temperature. Sometimes, people place an "X" in the wrong row or forget to mark it at all, leading to inaccurate temperature tracking.
-
Omitting actions taken during temperature excursions: If a temperature reading falls outside the acceptable range, specific actions must be taken, including documenting these steps on the Vaccine Storage Troubleshooting Record. Neglecting to fill out this part of the form can lead to insufficient responses to temperature breaches, potentially affecting vaccine viability.
Correctly maintaining the Refrigerator Temperature Log is essential for vaccine potency and safety. Paying attention to these common mistakes and ensuring accurate and thorough documentation can greatly contribute to the successful management of vaccine storage.
Documents used along the form
Managing proper storage and handling of vaccines is crucial to ensure their efficacy. Alongside the Refrigerator Temperature Log form, several other forms and documents play integral roles in facilitating optimal vaccine storage conditions. These documents help in maintaining the quality of vaccines, ensuring they remain effective for immunization purposes. Highlighted below are seven significant forms and documents often utilized in conjunction with the Refrigerator Temperature Log form:
- Vaccine Storage Troubleshooting Record: This document is essential for recording any storage anomalies affecting vaccine potency. It includes detailed information on the incident, the conditions of storage when the anomaly occurred, and the actions taken to mitigate the issue.
- Freezer Temperature Log: Similar to the refrigerator log, but specifically for freezer units, this log tracks the temperature within vaccine freezers to ensure it remains within the required range for frozen vaccines.
- Digital Data Logger (DDL) Report: A digital data logger provides precise and continuous temperature monitoring. The reports generated from a DDL offer a comprehensive overview of temperature conditions over time, highlighting any deviations that need attention.
- Vaccine Inventory Control Log: Effective vaccine management requires close monitoring of stock levels. This log helps track vaccine quantities, lot numbers, and expiration dates, ensuring timely use and replenishment of supplies.
- Corrective Action Plan: When temperature excursions occur, a corrective action plan outlines the steps to prevent future incidents. This document details the investigation findings, the root causes identified, and the corrective measures implemented.
- Emergency Vaccine Storage Plan: This document is vital for preparing for power outages or equipment failures. It outlines procedures for maintaining vaccine viability during emergencies, including alternate storage sites and contact information for immediate action.
- Staff Training Record: Ensuring that all personnel involved in the storage and handling of vaccines are adequately trained is crucial. This record documents completed training sessions, including dates and the subjects covered, to confirm competency in maintaining vaccine integrity.
Together, these documents support a comprehensive approach to vaccine management. By meticulously tracking temperatures, inventory levels, and addressing storage issues promptly, healthcare providers can safeguard vaccine potency, ensuring the success of immunization programs. Maintaining an organized record-keeping system, including the use of the Refrigerator Temperature Log and related documents, is instrumental in achieving these objectives.
Similar forms
Medication Temperature Log: Similar to the Refrigerator Temperature Log form, the Medication Temperature Log is used in medical facilities to record and monitor the temperatures at which medications are stored. Both forms serve a critical function in ensuring that vaccines or medications are stored within safe temperature ranges to maintain their effectiveness. Like the fridge log, this document typically requires recording temperatures at least twice per day and provides space for action taken if temperatures fall out of the recommended range.
Food Safety Temperature Log: This log is utilized in the food service industry to track the temperature of refrigerated and frozen food products to ensure they are stored safely and prevent foodborne illnesses. Despite the different contexts, both logs share the common goal of temperature tracking to ensure safety and compliance with health guidelines. They require regular entries and have protocols for addressing out-of-range temperatures, emphasizing the importance of immediate corrective actions.
Laboratory Sample Temperature Log: Laboratories use these logs to monitor the temperatures of refrigerators and freezers where samples are stored. Much like the Refrigerator Temperature Log for vaccines, this form is crucial for protecting sensitive biological samples from temperature deviations that could compromise their integrity. Both documents necessitate meticulous record-keeping and prompt responses to temperature excursions to preserve the viability of stored materials.
Server Room Temperature Log: In the tech industry, maintaining the right temperature in server rooms is vital to protect hardware from overheating and ensure system reliability. While this log tracks non-medical equipment, it requires the same diligent temperature monitoring and interventions for out-of-range conditions as the Refrigerator Temperature Log. Both forms play a critical role in preventing damage to their respective contents, demonstrating how essential temperature control is across various fields.
Dos and Don'ts
Maintaining the correct temperature in vaccine refrigeration is crucial for vaccine efficacy and safety. Here are essential dos and don'ts when filling out the Refrigerator Temperature Log form:
Do:- Monitor Temperatures Twice Daily: Ensure to document current temperatures twice each day to catch any discrepancies early.
- Use the Correct Temperature Monitoring Device (TMD): If available, use a digital data logger recommended for its accuracy and ability to record min/max temperatures.
- Document Min/Max Temperatures: Record the highest and lowest temperatures recorded at the beginning and end of each workday, enabling a comprehensive overview of temperature stability.
- Take Immediate Action on Out-of-Range Temperatures: Should temperatures fall outside the acceptable range, label exposed vaccine “do not use,” and follow the specified corrective procedures promptly.
- Save Each Month’s Log: Retain each month’s temperature log for three years or longer if required by state or local jurisdictions, ensuring compliance and audit readiness.
- Initiate Corrective Actions and Document: If encountering out-of-range temperatures, promptly record the incident, take immediate action, and document the measures taken in the Vaccine Storage Troubleshooting Record.
- Ignore Out-of-Range Alerts: Never overlook alerts or signs of temperature fluctuations that fall outside the 36ºF to 46ºF safe range.
- Discard Vaccines Without Proper Guidance: Do not discard exposed vaccines unless specifically instructed by your state/local health department or the manufacturer.
- Forget to Record Temperatures or Actions: Failing to document temperatures or corrective actions can lead to accountability issues and potentially endanger vaccine efficacy.
- Procrastinate on Equipment Checks: Neglecting regular checks on your temperature monitoring device and refrigerator can lead to unnoticed malfunctioning.
- Delay Reporting to Authorities: When temperatures deviate from the safe range, promptly reporting to the relevant health department or vaccine coordinator is critical.
- Overlook Preventative Measures: After resolving an incident of out-of-range temperatures, failing to take preventative measures for future occurrences risks the safety and effectiveness of the vaccines.
Misconceptions
There are several misconceptions surrounding the use of the Refrigerator Temperature Log form, especially in medical facilities where proper vaccine storage is critical. Understanding these misconceptions can help in correctly managing vaccine storage and handling requirements:
The form is only for COVID-19 vaccines: While the document mentions storage and handling of COVID-19 vaccines, it is essential for the management of all vaccines. The guidelines ensure that all vaccines are stored at temperatures that maintain their efficacy.
Logging temperatures is a one-time daily task: The form requires documenting temperatures twice daily. This ensures that any fluctuations can be caught and addressed promptly to avoid compromising the vaccine's potency.
Any staff member can manage the log: Although any trained staff member can fill in the log, it's crucial that personnel who understand the importance of vaccine storage and handling, and who are trained in corrective actions for out-of-range temperatures, manage the log. This ensures compliance with best practices.
Out-of-range temperatures are not urgent: If temperatures deviate from the recommended range, immediate action is necessary. The document outlines steps to label exposed vaccines, notify appropriate authorities, and document the incident thoroughly, illustrating the urgency of proper action.
Discarding vaccines is the first step after finding out-of-range temperatures: The form advises against discarding vaccines without authorization from local health departments or manufacturers. Often, vaccines may still be usable or require specific disposal procedures.
Keeping logs is only important for the short term: Facilities are instructed to save each month's log for three years or more, depending on state or local jurisdiction requirements. This long-term record-keeping is crucial for accountability, quality control, and compliance with regulatory standards.
Clarifying these misconceptions ensures proper storage and handling of vaccines, which is crucial for the effectiveness of immunization programs.
Key takeaways
Understanding how to accurately complete and utilize the Refrigerator Temperature Log for vaccine storage is crucial for maintaining vaccine efficacy and safety. Here are key takeaways to ensure proper management:
- Always record your initials and the exact time when checking the refrigerator temperatures to ensure accountability and tracking over time.
- Utilize a Temperature Monitoring Device (TMD), preferably a digital data logger, to record the minimum and maximum temperatures each workday. This helps in identifying any fluctuations that could affect vaccine potency.
- Mark the correct temperature row with an "X" to visually track the refrigerator's temperature status daily, aiding in quick detection of any deviations from the desired temperature range.
- If temperatures are found to be out of the safe range, immediate action is required, including labeling exposed vaccines as “do not use” and adjusting storage conditions as soon as possible.
- Document any out-of-range temperatures in the specified action area along with the room temperature to monitor potential environmental factors influencing storage conditions.
- Notify the appropriate vaccine coordinator or contact your state or local health department for guidance upon encountering any temperature discrepancies.
- It's important to complete the attached “Vaccine Storage Troubleshooting Record” to detail the incident, actions taken, and resolve the issue, preserving the integrity of the vaccine supply.
- Retention of each month's temperature log for a minimum of three years, or longer if mandated by state/local jurisdictions, is necessary for compliance and to facilitate any future audits or investigations.
- In case of exposure to out-of-range temperatures, do not discard vaccines until instructed by state/local health departments or the manufacturer, preserving potentially salvageable vaccines.
- Proactive measures should be put in place to prevent future occurrences, such as routine maintenance checks on storage units and contingency planning for power outages or equipment failure.
By adhering to these practices, healthcare facilities can ensure that vaccines remain potent and safe for use, ultimately protecting public health.
Popular PDF Forms
Supervision for Associates - By clarifying roles and responsibilities, it helps in establishing a professional and ethical foundation for future practitioners.
How Do X Rays Work - Used by pregnant women to provide X-ray history to OB/GYNs for precautionary measures during prenatal care.
Ldss 3370 - Scrutiny of the LDSS-3370 form by the agency liaison is crucial to catch and correct any errors or omissions before submission.